Abstract
The COVID-19 pandemic catalyzed the swift development and distribution of mRNA vaccines, including BNT162b2, to address the disease. Concerns have arisen about the potential neurodevelopmental implications of these vaccines, especially in susceptible groups such as pregnant women and their offspring. This study aimed to investigate the gene expression of WNT, brain-derived neurotrophic factor (BDNF) levels, specific cytokines, m-TOR expression, neuropathology, and autism-related neurobehavioral outcomes in a rat model. Pregnant rats received the COVID-19 mRNA BNT162b2 vaccine during gestation. Subsequent evaluations on male and female offspring included autism-like behaviors, neuronal counts, and motor performance. Molecular techniques were applied to quantify WNT and m-TOR gene expressions, BDNF levels, and specific cytokines in brain tissue samples. The findings were then contextualized within the extant literature to identify potential mechanisms. Our findings reveal that the mRNA BNT162b2 vaccine significantly alters WNT gene expression and BDNF levels in both male and female rats, suggesting a profound impact on key neurodevelopmental pathways. Notably, male rats exhibited pronounced autism-like behaviors, characterized by a marked reduction in social interaction and repetitive patterns of behavior. Furthermore, there was a substantial decrease in neuronal counts in critical brain regions, indicating potential neurodegeneration or altered neurodevelopment. Male rats also demonstrated impaired motor performance, evidenced by reduced coordination and agility. Our research provides insights into the effects of the COVID-19 mRNA BNT162b2 vaccine on WNT gene expression, BDNF levels, and certain neurodevelopmental markers in a rat model. More extensive studies are needed to confirm these observations in humans and to explore the exact mechanisms. A comprehensive understanding of the risks and rewards of COVID-19 vaccination, especially during pregnancy, remains essential.
Introduction
COVID-19, an infectious disease caused by the SARS-CoV-2 virus, has materialized as a cardinal global health crisis since its original detection in Wuhan, China in December 2019 [1]. In the wake of this pandemic, mRNA-based vaccines have been pioneered as a quintessential countermeasure to control viral dissemination and attenuate the disease severity. These vaccines, such as the Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) vaccines, operate by introducing mRNA molecules that encode the SARS-CoV-2 spike protein, subsequently inducing an immunogenic response [2, 3].
The spike protein, distinctly expressed on the surface of the SARS-CoV-2 virion, is instrumental in facilitating viral entry into host cells via interaction with the Angiotensin-converting enzyme 2 (ACE2) receptor [4]. Notwithstanding, apprehensions have been expressed about the plausible effects of the spike protein and its resultant immune response on the central nervous system (CNS). There are theoretical postulates suggesting that the biosynthesis of the spike protein, either through natural viral infection or post-vaccination, could induce neuroinflammation and elicit alterations in synaptic plasticity [5, 6]. These proposed changes might exert effects on brain development and have potential contributions to neurodevelopmental disorders, including autism [7].
Neuroinflammation, typified by the activation of CNS-resident immune cells and the subsequent release of pro-inflammatory cytokines, has been implicated in a gamut of neurodegenerative and psychiatric disorders [8, 9]. Various studies have demonstrated that viral infections, encompassing those triggered by the Coronaviridae family, can initiate a neuroinflammatory response [10]. The spike protein, owing to its interface with the ACE2 receptor, might cross the blood–brain barrier or indirectly induce neuroinflammation through peripheral immune signaling [11, 12].
In addition, there is burgeoning evidence suggesting that perturbations in synaptic plasticity—the inherent ability of synapses to strengthen or weaken over time in response to increases or decreases in their activity—may be implicated in neurodevelopmental disorders [13]. Preliminary preclinical research investigating the potential effects of the spike protein on synaptic function has delineated possible disruptions in neuronal connectivity and synaptic transmission [14, 15].
While the mRNA vaccines have exhibited exceptional efficacy in forestalling severe COVID-19 manifestations and curtailing viral transmission, it is of paramount importance to scrupulously investigate the potential neurological ramifications associated with the spike protein itself and with the immune response it induces [16, 17]. Gaining a comprehensive understanding of the implications of spike protein-induced neuroinflammation, along with its impact on synaptic plasticity and overall brain development, will bolster our knowledge of the long-term effects of COVID-19 infection and its vaccination [18].
In this study, our primary objective is to delve deeply into the existing literature and data concerning the potential relationship between COVID-19 mRNA vaccines, spike protein-mediated reactions, and the genesis of neurodevelopmental disorders, focusing on autism. We have chosen to measure specific cytokines as they are pivotal markers for neuroinflammation, providing insights into the inflammatory responses potentially triggered by the vaccine. Additionally, brain-derived neurotrophic factor (BDNF) is a key molecule involved in synaptic plasticity and neuronal health; changes in its levels can be indicative of alterations in synaptic plasticity, which may correlate with neurodevelopmental anomalies. We are also investigating the gene expressions of m-TOR and WNT due to their well-documented roles in neural development and synaptic function. To bridge the gap between molecular findings and potential clinical manifestations, we will employ behavioral tests designed to parallel autism-specific behaviors in humans, thereby enabling a comprehensive assessment of the vaccine’s potential neurodevelopmental implications. Through a detailed analysis of these carefully selected parameters, we aim to provide a clearer picture of this pressing area of research and offer directions for subsequent investigations [19, 20].
Materials and Methods
Animals
The Animal Ethics Committee (1823020911) of Demiroglu Science University gave its permission to the experimental techniques used in the current investigation. The Experimental Animal Laboratory of Demiroglu Science University provided the rats used in the study. The National Institutes of Health’s (U.S.) Guide for the Care and Use of Laboratory Animals was rigorously followed throughout all operations.
Wistar adult rats were used in the study; there were 15 females and 5 males, with an average weight of 220 ± 10 g. These animals were housed in plastic cages under conventional circumstances with an alternate 12-h light/dark cycle and an ambient temperature of (22 ± 2 °C).
Study Design
Two cohorts of female rats were randomly assigned to the following treatment groups: Group 1 or the 0.9% NaCl Saline Group (n = 7) and Group 2 or the COVID-19 m-RNA Vaccine BNT162b2 Group (n = 8). Throughout the experiment, the behavior and physical well-being of all animals were meticulously monitored daily. To facilitate the mating process, three female rats were cohabitated with a single male rat for a period spanning two to three days during the estrus phase. The presence of white vaginal plaque in the female rats was used as an indicator of successful mating. After this occurred, the male rats were removed from the enclosures.
Rats belonging to Group 1 were administered 1 ml/kg of 0.9% NaCl saline intramuscularly on the thirteenth day of gestation. Simultaneously, rats in Group 2 received a dosage of 30 µg/Rat of the COVID-19 m-RNA Vaccine BNT162b2 intramuscularly on the same day of pregnancy. To normalize maternal care, the number of pups per dam was culled to four on the day of parturition. The mothers were permitted to nurture their litters up until the point of weaning on postnatal day 21 (P21). Subsequently, on P21, a total of 41 progeny (comprising 10 male and 10 female saline-treated rats; along with 13 male and 8 female rats exposed to the COVID-19 m-RNA Vaccine BNT162b2) were systematically divided and accommodated in cages housing same-sex and same-study group members (Table 1). They had unrestricted access to standard food and tap water. Upon reaching maturity on P50, which we will refer to as Day 1 for clarity in the testing timeline, the rats began their behavioral assessments. Open Field Test: This initial assessment took place on Day 1. Serving as both a measure of general locomotor activity and anxiety, it also acclimated the rats to a testing environment. Novelty-Induced Rearing Behavior: On Day 4, the rats were evaluated for their vertical explorative behaviors in a novel setting. Three-chamber Sociability and Social Novelty Test: Administered on Day 7, this assessment provided insights into the rats’ sociability and their preference for social novelty. Their prior acclimation to the testing conditions by this point ensured accurate insights. Rotarod Test: On Day 10, the rats underwent this test to evaluate their motor skills and endurance. This physically demanding test was placed last in the sequence to minimize any fatigue or stress impacts on the outcomes of the earlier behavioral assessments. All behavioral examinations were conducted between 10:00 AM and 3:00 PM to ensure consistency in testing conditions and to control for potential diurnal variations in behavior. All behavioural evaluations were performed by video processing using an artificial intelligence-based behaviour analysis system and software (Scove Systems, http://scovesystems.com/, Izmir, Turkey).
Upon the termination of the study, all subjects were euthanized via cervical dislocation. Their brains were subsequently extracted for the purpose of comprehensive biochemical and histological evaluations. The procedure was carried out under anesthesia composed of Ketasol (100 mg/kg, manufactured by Richterpharma AG, Austria) and Rompun (50 mg/kg, produced by Bayer, Germany).
In our study, an observable difference in the number of male and female pups within the COVID-19 mRNA Vaccine group was noted, as presented in Table 1. This variance stemmed from the natural sex distribution in the litters and not from any specific selection criteria employed during our experimental procedures. To ensure a consistent environment for maternal care, we implemented a culling protocol where each dam was allowed to rear a set number of four pups post-parturition. The selection process for culling was based solely on maintaining this pup number and was not influenced by the sex of the offspring. Consequently, the resulting total of 13 viable male pups and 8 viable female pups in the COVID-19 mRNA Vaccine group reflected the natural variation in the sex ratio of the litters. This natural variability was further evidenced by the comparable average litter sizes observed between the control group (% 0.9 NaCl Saline) with an average of 8.5 pups and the COVID-19 mRNA Vaccine group with an average of 8.2 pups. It is important to clarify that these numbers were a product of the natural breeding patterns and not a result of our experimental design or interventions.
Behavioral Tests
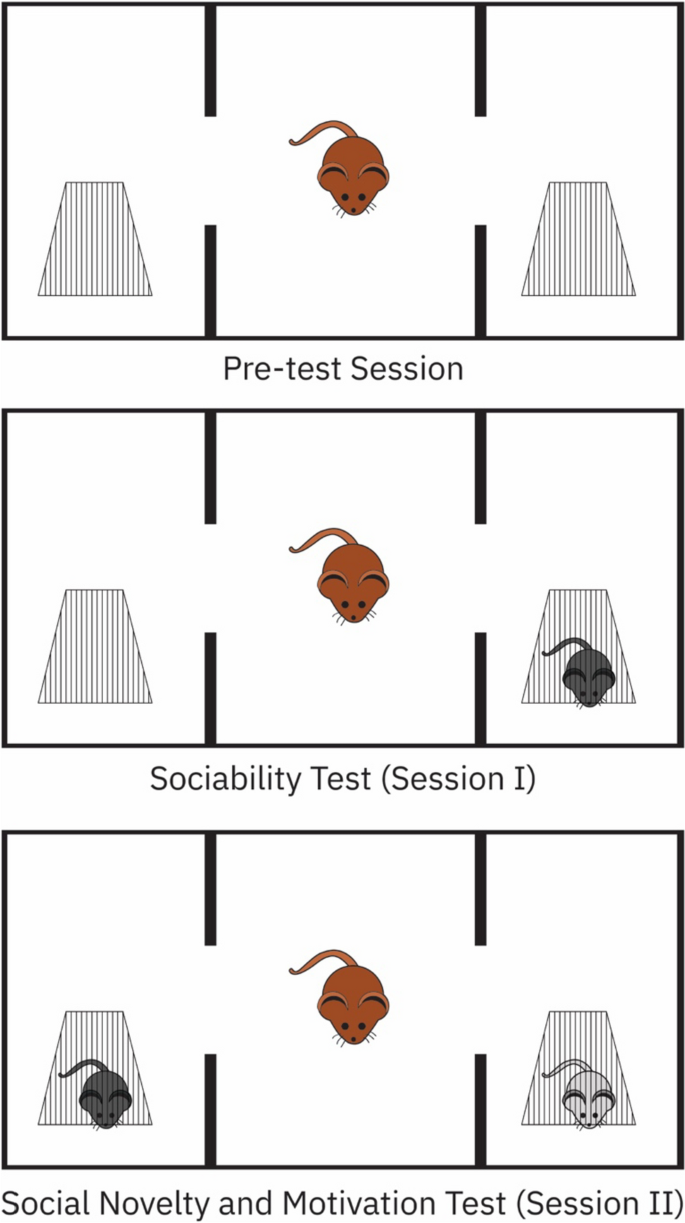
Three-Chamber Sociability and Social Novelty Test
Our procedure involved slight modifications from previously established protocols [21,22,23]. We used an opaque black Plexiglas enclosure, measuring 40 cm × 90 cm × 40 cm, which was sectioned into three equal chambers, each measuring 40 cm × 30 cm × 40 cm (Fig. 1).