All Global Research articles can be read in 51 languages by activating the “Translate Website” drop down menu on the top banner of our home page (Desktop version).
To receive Global Research’s Daily Newsletter (selected articles), click here.
Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
Global Research Editor’s Note
Read carefully. Pfizer is committing crimes against humanity, specifically against our children.
The vaccine has resulted in cardiac arrest not in an elderly person but in a two month old baby.
“Why did they not follow up on the 2-month-old baby’s condition, after going into cardiac arrest an hour after receiving an experimental vaccine? Why is there no further information? Is it because he died? Or was the baby removed from an experiment? Why would the author of the report not mention this?”
We call upon the US Department of Justice to undertake a criminal investigation against Pfizer.
We call upon governments worldwide to immediately suspend the mRNA vaccine.
A class action law suit is also required on behalf of the hundreds of thousands of victims of the mRNA vaccine.
Never mentioned by the media, Pfizer has a criminal record with the US Department of Justice.
In 2009 Pfizer was indicted on charges of “fraudulent marketing”.
Michel Chossudovsky, Global Research, July 6, 2022
***
An analysis of VAERS reports shows that contrary to the FDA’s briefing document claiming that the majority of adverse events in Pfizers’ clinical trial were non-serious – at least 58 cases of life-threatening side effects in infants under 3 years old who received mRNA vaccines were reported. For some, it is unclear if they survived. It is also unclear why the infants were vaccinated, and whether they were part of the clinical trials. However, in the upcoming FDA meeting on Wednesday, the FDA will not be able to argue it did not know
“Chest pain; cardiac arrest; Skin cold clammy”. This short description of a cardiac arrest, which occurred one hour after receiving a Pfizer-BioNTech COVID-19 vaccine, is taken from the VAERS system – the US Vaccine Adverse Eve Reporting System ( case number 1015467), and it does not refer to an elderly person, nor to a young adult, or even a teenager. It is hard to believe, but this report refers to a two-month-old baby.
“A 2-month-old male patient received bnt162b2 (PFIZER-BioNTech COVID-19 VACCINE) lot number: EL 739, via an unspecified route of administration on 02 Feb 2021 at single dose for COVID-19 immunisation”, thus stated in the report. “Patient administered vaccination, observed for 15 minutes left the clinic then returned one hour later on 02 Feb 2021, presenting as skin cold, clammy and with chest pain, cardiac arrest event then developed, patient stabilised and transferred for further medical treatment… The outcome of the events was unknown. This case was reported as serious with seriousness criteria-life threatening from HA. No follow-up attempts possible. No further information expected”.
How did a 2-month-old baby receive the mRNA vaccine? These vaccines have not yet received EUA (Emergency Use Authorization) for approved use in children ages five and under by the FDA, or any other regulatory authority, and even if it will, the EUA will only include babies 6 months and older.
Was this baby a participant in Pfizer-BioNTech’s clinical trials, testing efficacy and safety among babies?
The answer is unclear. According to the person who wrote the report “Unsure if patient was enrolled in clinical trial”. However, the author of the report also states that the report was ”received from a contactable Other Health Care Professional by Pfizer from the Regulatory Agency”. This note implies that the infant might have actually participated in Pfizer’s trial. The regulatory agency report Safety Report Unique Identifier GB-MHRA-ADR 24687611 – indicates that the report came from Great Britain (the first 2 letters in the report ID stand for the country of origin, GB- Great Britain, and MHRA indicate that the source of reporting was its’ drug authority).
Why did they not follow up on the 2-month-old baby’s condition, after going into cardiac arrest an hour after receiving an experimental vaccine? Why is there no further information? Is it because he died? Or was the baby removed form an experiment? Why would the author of the report not mention this?
Shockingly, it turns out that this incident is not isolated, but in fact one of many in the VAERS system, describing babies and children under five exposed to mRNA Covid vaccines, who suffered life-threatening adverse reactions.
Even though children under five were not considered eligible for these vaccines unless they were part of a clinical trial, astonishingly, it appears that there are many reports in the system describing babies and toddlers who were vaccinated. Some of the children suffered from life-threatening adverse events. In some cases, it is not clear what happened to them; did they survive and recover, do they still suffer from health problems, or did they die.
In a couple of days, on June 15, the FDA’s Vaccines and Related Biological Products Advisory Committee will discuss Moderna and Pfizer’s EUA requests for vaccines for infants and toddlers aged 6 months to 4 years – the only group not yet eligible for COVID-19 vaccination today.
According to the FDA’s briefing document released today ahead of the VRBPA committees’ meeting, there were “245 US reports” to the VAERS system “in children 6 months through 4 years of age”, who were injected (“product administered to patient of inappropriate age” or “off-label use”) or exposed to the vaccine “via breastmilk”. Nevertheless, both companies announced already in May that their findings indicate that their vaccines are safe and effective.
The VRBPAC Briefing Document lists a variety of adverse events reported following the exposure to the vaccine in this age group, including “pyrexia…, body temperature…, cough, headache, rash, diarrhea”. According to the document, “Among US VAERS reports for individuals aged 6 months through 4 years, which may reflect unauthorized use of the vaccine or may reflect a reporting error, the majority (96.3%) were non-serious”.
While the document specifies safety concerns identified from post-authorization safety surveillance data in VAERS, including anaphylaxis, myocarditis, and pericarditis, it does not relate to these safety concerns identified in the younger age group. Instead, it states: “No unusual frequency, clusters, or other trends for adverse events were identified that would suggest a new safety concern”.
But is that really the case? It seems that regardless of the results, and despite the disturbing and shocking findings that are being exposed from Pfizer’s documents, it is expected that both companies will receive the desired EUA very soon. In fact, the CDC website, already in April, had advertised a protocol regarding children’s vaccination, which included babies 6 month to 4 years as well.
In light of this expected approval, RT Magazine conducted an analysis of the cases reported in the VAERS system referring to babies up to 3 years old.
During the analysis, cases were removed in which it was stated that the exposure to the vaccine was through breastfeeding (these cases were analyzed separately and will soon be presented in a follow-up article), as well as cases that were identified as errors in the age registration.
The analysis shows there were at least 58 cases of severe and life-threatening adverse reactions among babies and toddlers 3 years old and younger. This finding is especially puzzling considering the fact that they weren’t supposed to be vaccinated at this age to begin with. Sadly, similarly to the case reported above, most VAERS reports do not indicate how and under which circumstances they were exposed to the vaccine – were they participants in the companies’ trials? And if not, why and in which circumstances were they vaccinated?
Both companies have not yet released the safety data from their trials on this age group. However, one thing is clear from the VAERS reports: there were many babies who were injured after receiving the vaccine. Whether they were vaccinated in the trials or illegally in their communities, Pfizer and Moderna will defiantly not be able to claim, when presenting their data to the FDA, that the vaccine is safe for babies, and that there weren’t any severe adverse events in this age group. Moreover, the FDA’s committee experts who will discuss the EUA approval will not be able to ignore those cases and argue that they did not know. The data presented in this article demonstrate beyond any doubt the complete opposite, and this time – these data are presented to the public in advance, before the EUA is granted and ahead of the VRBPAC discussion.
The outcome of the events: Did not recover
One of the most chilling reports refers to a 43-day-old female baby, who on January 30, 2021, received Pfizer’s Comirnaty vaccine. In the incident description (report no. 1133837) it is clearly stated that she was vaccinated and that the vaccine was injected to the muscle: “A 43-days-old female patient received bnt162b2 (COMIRNATY), intramuscular on 30Jan2021 (Lot Number: EK9788) as SINGLE DOSE for COVID-19 immunisation”. Right after the vaccination, the baby suffered a variety of life-threatening multi-system injuries, such as “Anaphylactic reaction (broad), Asthma/bronchospasm (narrow), Anticholinergic syndrome (broad), Acute central respiratory depression (broad), Pulmonary hypertension (broad), Cardiomyopathy (broad), Eosinophilic pneumonia (broad), Vestibular disorders (broad), Hypersensitivity (broad), Respiratory failure (narrow), Drug reaction with eosinophilia and systemic symptoms syndrome (broad)“. Although in the section reporting death the statement states “No”, the section reporting recovery also states “No” – meaning the baby has not recovered. What then happened to her? Is she alive or did she die?
In addition, this report, like many others, raises some difficult questions. How did a 43-day-old baby receive a vaccine not yet approved for use in babies? Furthermore, the current clinical trials conducted are supposed to include babies and children over 6 months. Was this baby a participant in Pfizers’ trial? The report does not answer to this question.
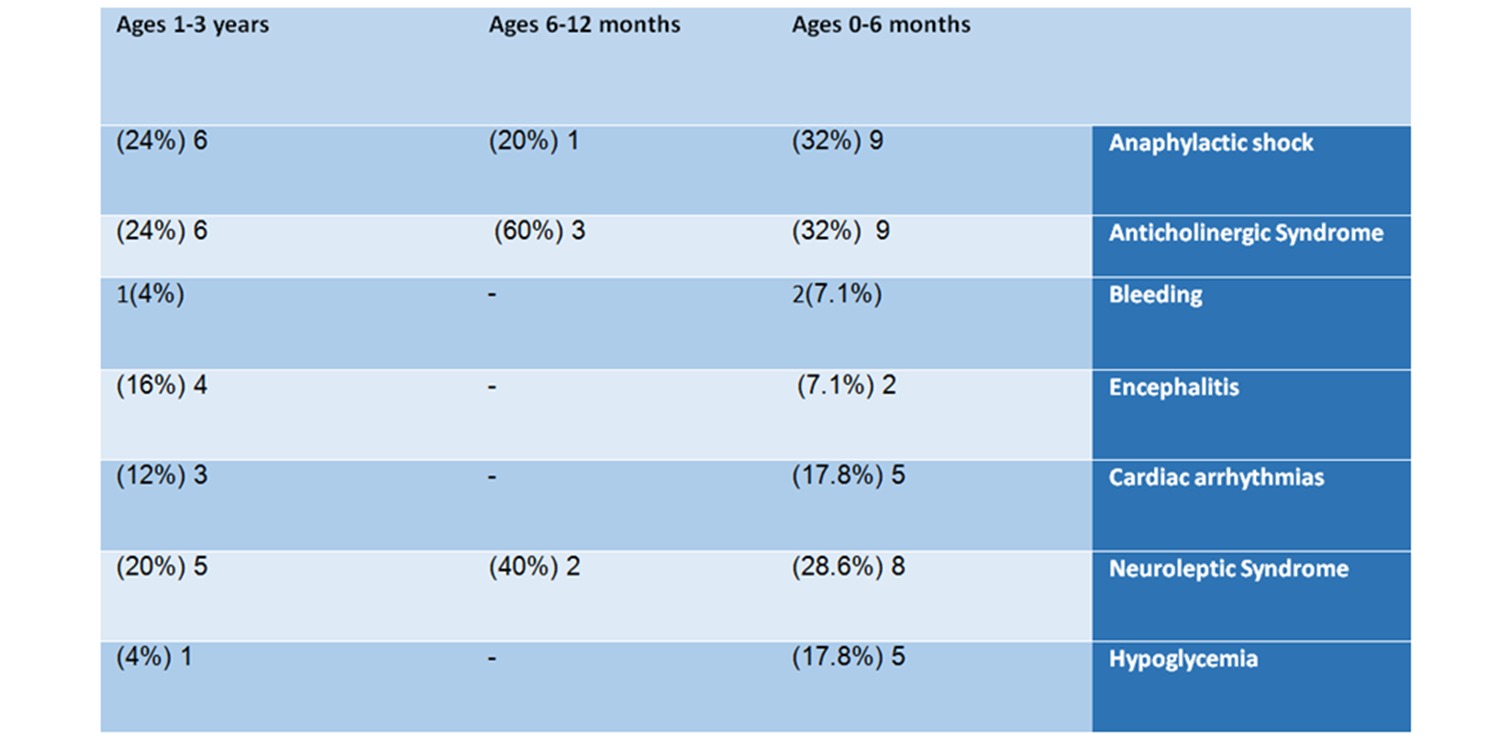
Just like this baby, it turns out that in most of the reported cases several life-threatening side effects were recorded for the same baby. The most common severe adverse events were dangerous hemorrhaging; anaphylactic shock – a life-threatening allergy that can damage the respiratory system and cause dizziness, fainting, and even death; anticholinergic syndrome- a condition that occurs when the receptor sites for the neurotransmitter acetylcholine are blocked, which can lead to coordination problems, increased heart rate, and other symptoms; encephalitis – a brain infection, that can cause headaches, vomiting, loss of consciousness and death; hypoglycemia – very low blood sugar, a condition that can quickly escalate to death in infants; and neuroleptic syndrome – which is also life-threatening , and can damage the heart muscles, other muscles, and the kidneys.
From the summary of the findings of the analysis according to age and gender groups, the following picture emerges:
In the age group of 0-6 months – there are 28 reports, in which 10 are males, 16 are females, and 2 whose gender was not specified.
9 of them (32%) suffered an anticholinergic syndrome, 9 (32%) had an anaphylactic shock, 8 (28.6%) suffered Neuroleptic syndrome, 5 suffered from heart rhythm irregularities, and 5 had hypoglycemia.
In the age group of 6-12 months – in this group, 5 reports were found – 3 males, one female, and one whose gender was not specified. This group is small compared to the other groups. The list of adverse reactions included: anaphylactic shock, anticholinergic syndrome, and Neuroleptic syndrome.
In the age group of one-to-three year old – in this group 25 cases were reported, of which 5 related to males, 19 related to females, and one to a baby whose gender was not specified.
6 of the babies (24%) had an anaphylactic shock, 6 (24%) suffered anticholinergic syndrome, 5 (20%) suffered from Neuroleptic syndrome, 4 (16%) suffered encephalitis, 3 (12%) had irregular heartbeats, one baby was hemorrhaging and one suffered from hypoglycemia.
It should be noted that the adverse events listed above are only some of the ones reported in VAERS with respect to babies. We have chosen to focus only on life-threatening and common adverse events.
Table No. 1: Analysis of reports by age and gender
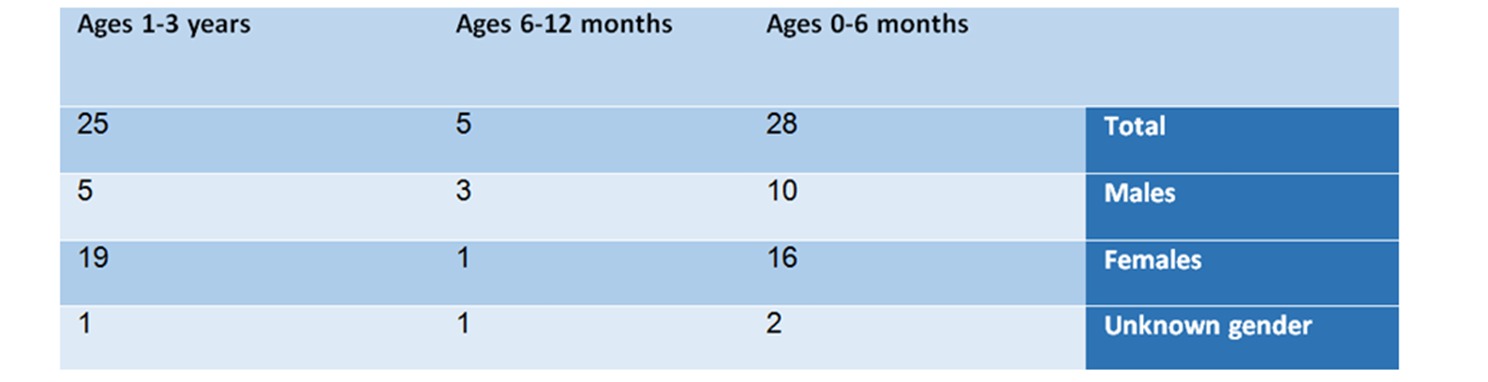
Table No. 2: Analysis of reports by adverse events
Are the babies alive?
Similarly to the previous case described, another baby, two months old, also went through anaphylactic shock after being exposed to a single dose of the Pfizer-BioNTech vaccine on January 6, 2021, and just like her, according to the report (no. 976433), she suffered from an array of multi-system symptoms. Regarding the method of administering the vaccine, it was stated ”via an unspecified route of administration”, meaning it is not clear in what circumstances the baby was exposed to the vaccine.
Was she part of Pfizers’ clinical trial? Again, it is unclear from the report.
However, the more important question that should be asked, just like in the previous case, is what happened to the baby? Did she survive? Is she alive?
And again, in the section reporting death, it states “No”, meaning the baby did not die. However, in the report description it says, “The patient had not recovered from the event. No follow-up attempts possible. No further information expected”.
It is hard to believe, but this basic question – what happened to a baby after suffering such severe and life-threatening adverse reactions – also arises from other serious cases, such as the case of a 6-month-old baby (report # 2084418) who “received bnt162b2 (COMIRNATY), intramuscular” on December 29, 2021, and went through anaphylactic shock, anticholinergic syndrome, Neuroleptic syndrome, infectious pneumonia, other infections, and multi-system symptoms.
In this case as well, the section reporting death states “No”, meaning supposedly the baby did not die, while in the event description it says “outcome ‘unknown’… No follow-up attempts are possible. No further information is expected”.
In another case (report no. 1012508) a one-year-old baby who also received a Pfizer vaccine, in January 19, 2021 (this case it is specified that the baby did not take part in a trial) developed a pain in her left ear that escalated to full paralysis, which was diagnosed as Guillain Barre syndrome. In the case description it was stated that the baby suffered Guillain Barre Syndrome, face paralysis, non-infectious encephalitis, non-infectious meningitis, earaches and hearing disorders. Nonetheless, in the summary of the report, it was written, again, that “No follow-up attempts are possible”.
And another shocking case (report number 1379484) emerges from the report of a baby who was only one month old, who suffered “Vaginal bleeding/ Constant heavy vaginal bleeding with chunks of clot” the following day after receiving the Pfizer-BioNTech vaccine on May 19, 2021.
Although the symptoms the baby suffered from were defined as “serious as medically significant”, in the incident description it is stated that the result is “unknown” and that “No follow-up attempts are possible. No further information is expected”.
As mentioned, in some of the cases it is stated the babies were not part of a clinical trial, while in others it is not clear whether they participated in a clinical trial or were vaccinated in other unknown circumstances. But whether they were part of the trial or not, the report does not explain the absence of this critical information; what happened to these babies? Did they survive? And if so, did they recover? Why was there not a follow-up on the medical condition of babies who suffered from severe and life-threatening adverse events, while it was clearly stated that they did not recover? Is it not required in such severe cases by the FDA that the company should make every effort to locate these babies, find out what their condition is and follow up on them?
“Redness in the injection area: the clinical trial protocol does not mention severe adverse reactions”
The press release issued in February 11, 2022, in which Pfizer-BioNTech announced that they intend to apply to the FDA for approval for infants from 6 months to 4 years of age, the safety findings from the company’s clinical trials in babies and toddlers at these ages are not mentioned, not even in a word. The information brochure regarding the clinical trials testing the safety and efficacy of the Pfizer vaccine in adults, children and babies, on the FDA website, clearly states “No Study Results Posted on ClinicalTrials.gov for this Study”. And as noted above, the newly released The VRBPAC Briefing Document only lists a handful of non-serious adverse events reported in this age group, including, and concludes that there is nothing that would suggest a new safety concern. How could the FDA not know about so many serious adverse events that were reported to the CDC’s reporting systems? Alternatively, if they do know about them – why are they ignoring them?
How were adverse events in babies tested than in the clinical trials? In an attempt to answer this critical question, intended to address the safety issues and to assure parents that the vaccine is safe for babies, we examined the study protocol found on the FDA clinical trial website.
It appears that no potential severe adverse events were listed. The list of potential adverse events that the study was supposed to evaluate according to the protocol (“outcome measure”) did include both local and systemic reactions. However, these are relatively non-serious adverse events.
The list of local adverse events that the trial was supposed to monitor includes: “Pain or tenderness at the injection site, redness and swelling”, and the systematic reactions included ”Fever, fatigue, headache, chills, vomiting, diarrhea, new or worsened muscle pain, new or worsened joint pain, decreased appetite, drowsiness, and irritability”. Moreover, although the study is scheduled to end only on June 14, 2024, the time frame set for examining adverse events is limited to seven days after each of the doses – the first and the second dose.
The vaccine is ineffective in infants. The solution: lower the efficiency threshold and add a third dose
In addition to the substantial concerns regarding the vaccines’ safety for babies, their efficacy in this age group is questionable by and large. According to the available data, healthy children are at almost zero risk for severe illness, hospitalization, or death due to COVID-19.
Hospitalization due to COVID-19 is very rare among children, and death cases are even rarer. In Germany, for instance, a large study found that not even one child died of COVID-19 among 5-11 age group without pre-existing conditions. Under these circumstances, even one case of a serious adverse event, let alone death, is crucial and outweighs any possible benefit of the vaccine.
Not surprisingly, Pfizer clinical trials in babies under 4 proved that 2 vaccine doses do not increase their antibody count significantly. The FDA commissioner, Dr. Janet Woodcock, admitted in an interview in early April 2022 that “The antibodies that were developed were not as high, so they didn’t have the same antibody response to the two-shot series in the older kids. It wasn’t as high as what we would have hoped for the younger as it was for the older kids”. According to Woodcock, this is why Pfizer, which planned to apply for EUA approval for babies in February, postponed the submittal date and decided to add a third dose to the trial and wait for the findings after all babies got their third dose.
Furthermore, in a statement given on May 11, Dr. Peter Marks, director of the Center for Biologic Evaluation and Research at the FDA, announced that infant and toddler vaccines will not need to pass the 50% efficacy rate against Covid. A 50% efficacy rate is the threshold adult vaccines need to pass. However, Marks explains that despite the previous guidelines, the FDA will not deny companies now approval for babies and toddlers just because it did not reach the 50% efficacy in preventing symptomatic infections.
Pfizer issued a press release on May 23 announcing that “Vaccine efficacy of 80.3% was observed in descriptive analysis of three doses during a time when Omicron was the predominant variant”. According to the press release, “The study suggests that a low 3-ug dose of our vaccine…, provides young children with a high level of protection against the recent COVID-19 strains”.
Yet, the FDA’s briefing document reveals that the claim for a “high level of protection” is based on a total of 10 symptomatic cases of COVID-19 identified in the trial, that occurred at least 7 days postDose 3. Three of them occurred among participants 6-23 months of age (which included 555 participants – 376 in the vaccine group and 179 in the placebo group) – with 1 case in the Pfizer-BioNTech vaccine group and two in the placebo group. Seven other cases occurred among participants 2-4 years of age (which included 860 participants – 589 in the vaccine group and 271 in the placebo group) – with 2 cases in the Pfizer-BioNTech vaccine group, compared to 5 in the placebo group. Nevertheless, the vaccine’s efficacy was framed by the FDA as 80,4%, and the document concludes that “Available data support the effectiveness of the Pfizer-BioNTech COVID-19 Vaccine 3-dose primary series (3 µg each dose) in preventing COVID-19 in the age group of 6 months through 4 years”. In addition, the document states that “Among infants and children 6 months through 4 years of age, rates of hospitalization and death due to COVID-19 are higher than among children and adolescents 5-17 years of age, and comparable to individuals 18-25 years of age, underscoring the benefit of an effective COVID19 vaccine in this age group”.
How ethical is it to give a baby a vaccine for a disease that the chances of getting severely ill or dying from it is almost zero, while the benefits of the treatment are unclear and, and life-threatening adverse reactions are very significant?
This question was the topic of an article published in March this year in Bioethics. The researchers stated that not even one of the main claims argued to justify approval for babies is valid. According to them, the benefits of the vaccine for healthy children are minimal, and therefore, even though complications are rare, they outweigh the vaccine’s benefits, especially since it is highly unclear what the short and long-term risks are, and the experience with the vaccine is very short. The altruistic claim of protecting the environment is also very problematic, since as a vaccine exists, the groups at risk can defend themselves, and it was proven already that children are not the main transmitters of the virus.
Congress members demand answers
This ethical issue has been raised in recent days by 18 members of Congress in a letter issued to the FDA on June 7, demanding answers before the authority’s decision to grant an emergency permit for the infant vaccine. Members of Congress demanded to know why COVID-19 vaccines are necessary for this age group in light of the fact that the disease poses a very small risk to infants and young children, that vaccines have little efficacy, and that there are many unanswered questions regarding these vaccines’ safety and adverse events.
The letter presents 19 questions to the FDA, including, among others – why did the FDA delay the publication of the hundreds of thousands of data pages from the manufacturers’ studies, the state of adverse events, and when can all FDA data be expected to be made public? The FDA was also asked to provide the public with more details regarding children who were severely injured or died from COVID-19, and how many children in general became seriously ill. Legislators also addressed the issue of cardiac risks in giving the mRNA COVID-19 vaccines to children, noting that following vaccinations given to large numbers of children aged 5-18, an increase in myocarditis and pericarditis was observed, with some cases ending in death, and the long-term effects of heart-related inflammation not yet quantified by health authorities. What’s more, lawmakers demanded to know why the FDA lowered the threshold of efficacy for the vaccines specifically for infants and youngest children, thus actually allowing companies to apply for EUA without any justification.

The FDA will not be able to argue it did not know
As stated, the data emerging from the analysis presented in this article demonstrate beyond any doubt that the vaccine is not safe for babies and toddlers. Whether these children were part of the study or not – these reports have been in the VAERS system for many months, so there is no chance that the FDA does not know them. Unfortunately, the fact that the FDA was aware of at least some of the serious adverse events, including increased risk of morbidity in the first days after vaccination, myocarditis, and increased risk of miscarriage and fetal malformations, and yet approved the vaccine for teens, children, and pregnant women, was later revealed too late – long after the EUA was granted to Pfizer and Moderna, when many have already been harmed. It only became clear thanks to FOIA (Freedom of Information) requests submitted to the FDA and other health authorities, and only after the FDA was forced by the court to disclose the documents. This time, the VAERS data presented here makes it possible to reveal this fact even before the approval. The FDA will not be able to claim that it did not know.
*
Note to readers: Please click the share buttons above or below. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.