Ivermectin is an anti-parasitic agent that has gained attention as a potential COVID-19 therapeutic. It is a compound of the type Avermectin, which is a fermented by-product of Streptomyces avermitilis. Bifidobacterium is a member of the same phylum as Streptomyces spp., suggesting it may have a symbiotic relation with Streptomyces. Decreased Bifidobacterium levels are observed in COVID-19 susceptibility states, including old age, autoimmune disorder, and obesity. We hypothesize that Ivermectin, as a by-product of Streptomyces fermentation, is capable of feeding Bifidobacterium, thereby possibly preventing against COVID-19 susceptibilities. Moreover, Bifidobacterium may be capable of boosting natural immunity, offering more direct COVID-19 protection. These data concord with our study, as well as others, that show Ivermectin protects against COVID-19.
Introduction
SARS-CoV-2 infection has been a global pandemic affecting the world for the last 2+ years. Symptoms include fever, cough, shortness of breath, GI issues, potential pneumonia, and other less common ones, including oral symptoms (Khodavirdipour et al., 2021a; Sharma et al., 2021). Current treatments for SARS-CoV-2 include remdesivir, paxlovid, hydroxychloroquine, nucleoside analogs, antibodies, antibiotics, herbal medicines, tocilizumab, anti-inflammatory drugs (e.g., steroids), and ivermectin (IVM) (Gavriatopoulou et al., 2021). Vaccines have been distributed widely for SAR-CoV-2 infection (Khodavirdipour et al., 2020; Joshi et al., 2021) prevention. In addition to standard nasopharyngeal tests, CRISPR-based methods (Khodavirdipour et al., 2021c) have been used or proposed for SARS-CoV-2 infection testing. Recent mutations have been making SARS-CoV-2 infection more contagious and with a higher rate of Khodavirdipour et al. (2021b) infection. Nonetheless, the pandemic has continued for over 2 years, and a better understanding of all possible therapeutics is essential.
Ivermectin Has Been Shown to Effectively Treat SARS-CoV-2 Infection
Over 40 peer-reviewed studies (Table 1) have demonstrated studies on the effectiveness of IVM in SARS-CoV-2 infection
Thus, there is a tremendous need to ascertain, not whether but how IVM is ideally applied for SARS-CoV-2 infection.
The demonstrated effectiveness of IVM in SARS-CoV-2 cannot be ignored. An important step toward refining our understanding of when and how IVM is successful is that we need to understand all potential mechanisms for IVM against SARS-CoV-2 infection. The purpose of this study is to describe a novel hypothesis for IVM action against SARS-CoV-2 for consideration by the scientific community. While there is limited published evidence for this—as of yet theoretical description—we are soon to publish data showing in vivo administration of IVM increases-relative abundance of Bifidobacterium.
Background to the Hypothesis
IVM discovery was awarded the Nobel prize (Molyneux and Ward, 2015), 35 years post-discovery, for its game-changing impact on the field of antiparasitic agents. IVM is approved by the Food and Drug Administration for treating parasitic infections and comes from the class compounds called macrocyclic lactones, specifically Avermectins. Avermectins are found naturally as a fermentation product of a strain of Streptomyces avermitilis While IVM’s mechanisms of action as an anti-parasitic agent are well-established (Ômura and Crump, 2014), its potential in fighting viral infectious disease, including possibly SARS-CoV-2 infection, remains poorly understood.
Streptomyces spp. belong to the same phylum as a critically important constituent of the gut microbiome and common ingredient of probiotics, Bifidobacterium. Bifidobacterium abundance is known to decrease in SARS-CoV-2-infected subjects, as seen in ours and other studies (Tao et al., 2020; Xu et al., 2020; Reinold et al., 2021; Yeoh et al., 2021; Zuo et al., 2021; Hazan et al., 2022b). We have anecdotally observed, through our clinical experiences pre and post Fecal Microbiota Transplant, that certain bacteria in the same phylum may be able to replace each other’s function. Thus, we hypothesized Streptomyces spp. may replace loss of Bifidobacterium.
Bifidobacterium spp. are microaerotolerant anaerobes that degrade monosaccharides, such as glucose and fructose, via the bifid shunt, or the fructose-6-phosphate phosphoketolase pathway and produce more ATP than traditional fermentative pathways (De Vuyst et al., 2014). Bifidobacterium spp. also symbiotically feed other gut microbes via their metabolic by-products and end-products, which, in turn, enhance butyrate production and reduce inflammation (De Vuyst et al., 2014). Streptomyces spp., too, are frequently found in symbiotic relations with other bacteria (Seipke et al., 2012). Thus, it is possible for a symbiotic relation between Streptomyces and Bifidobacterium.
Increased Bifidobacterium levels serve as an important indicator of human health and may promote anti-inflammatory activity . It is interesting to note that these same disorders are key risk factors in severe SARS-CoV-2 infection, and Bifidobacterium abundance is also shown to be low in SARS-CoV-2 infection. Probiotics, which typically contain much Bifidobacterium spp., have been proposed as potentially useful SARS-CoV-2 infection prophylaxis or treatment
The mechanisms for Bifidobacterium boosting “natural immunity,” thereby protecting against SARS-CoV-2 infection effects, may involve its anti-inflammatory properties Specifically, Bifidobacterium increases Treg responses and reduces cell damage by decreasing the function of TNF-α (the pro-inflammatory “master-switch,” see and macrophages. Bifidobacterium spp. also increase the anti-inflammatory cytokine interleukin (IL)-10, regulate the helper T cell response , and, in a mouse model of inflammatory bowel disease, B. bifidum and B. animalis reduced pro-inflammatory cytokines and restored intestinal barrier integrity (i.e., decreased potential for “leaky gut”) . In short, Bifidobacterium, through TNF-α and interleukins, can decrease inflammation, leading to increased immunity. Moreover, a decrease in pro-inflammatory cytokines and increase in anti-inflammatory ones can help negate the cytokine storm of SARS-CoV-2 infection.
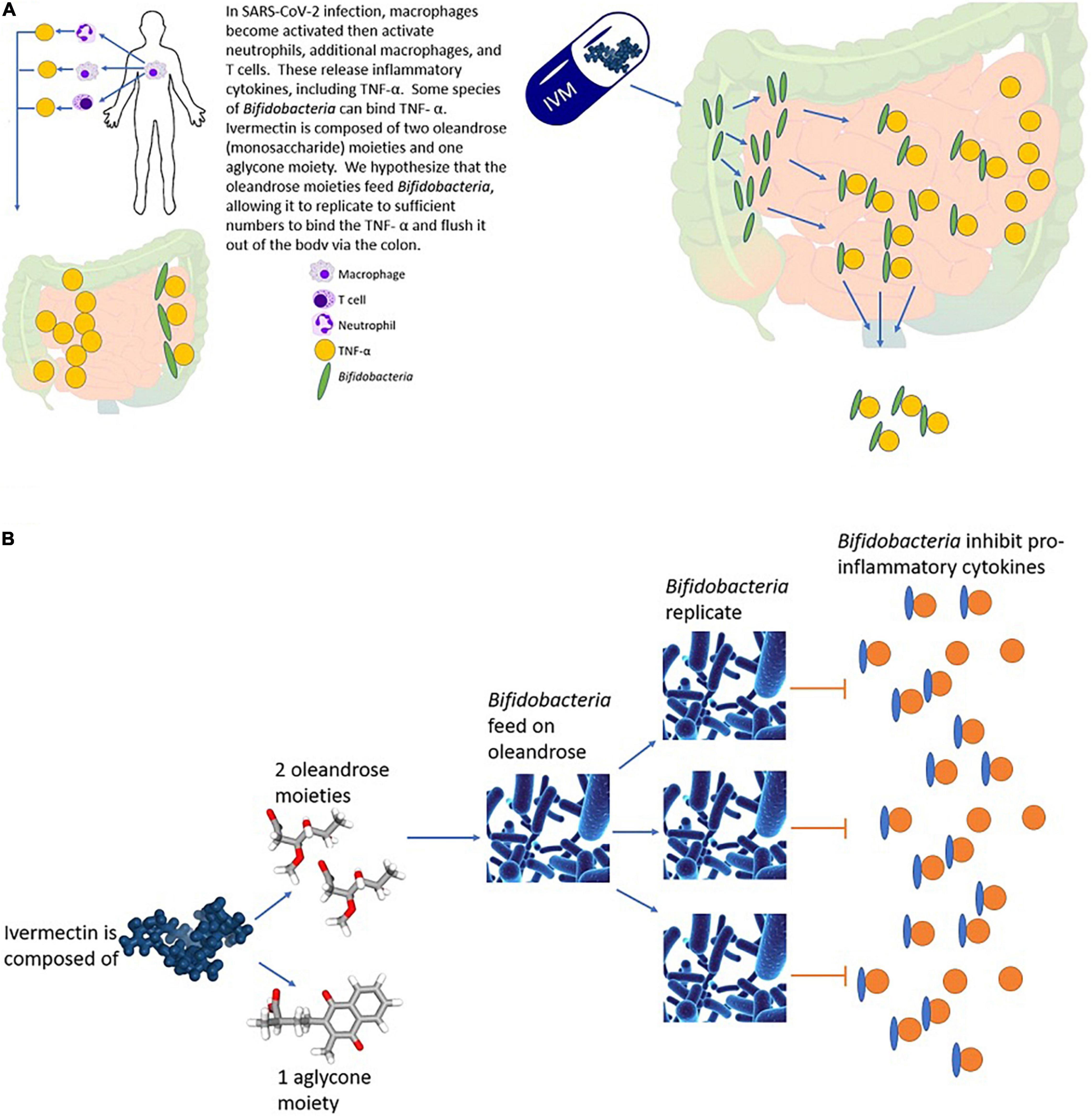 FIGURE 1. We hypothesize that IVM treats SARS-CoV-2 infection by decreasing inflammation through enhanced replication of Bifidobacterium, leading to inhibition of TNF-α. (A) Bifidobacterium inhibits inflammation via binding of TNF-α. (B) Expansion of the right side of panel (A), showing IVM contains monosaccharide oleandrose moieties that could feed Bifidobacterium, thereby increasing replication and increasing inhibition of TNF-α and inhibition of inflammation.
FIGURE 1. We hypothesize that IVM treats SARS-CoV-2 infection by decreasing inflammation through enhanced replication of Bifidobacterium, leading to inhibition of TNF-α. (A) Bifidobacterium inhibits inflammation via binding of TNF-α. (B) Expansion of the right side of panel (A), showing IVM contains monosaccharide oleandrose moieties that could feed Bifidobacterium, thereby increasing replication and increasing inhibition of TNF-α and inhibition of inflammation.
Hypothesis and Evaluation
Bifidobacterium is a heterotroph that feeds on a variety of carbon sources, but, most efficiently, simple sugars (oligosaccharides and monosaccharides) ). IVM is composed of two oleandrose (a monosaccharide) moieties, along with one aglycone moiety. A naturally found reversible glycosyltransferase, AveBI, can breakdown or assemble IVM to or from these components . Thus, IVM breakdown products include oleandrose, a monosaccharide that may feed Bifidobacterium, thereby promoting its growth
There may be other mechanisms of action of IVM in SARS-CoV-2 infection treatment. Studies found that IVM has significant potential binding affinity for many proteins within SARS-CoV-2 ). Ivermectin could, thus, block the binding of the spike protein’s receptor binding domain within SARS-CoV-2 to the ACE2 receptor This binding between the spike protein and ACE2 receptor is essential for viral entry. This same study also demonstrated that IVM has the ability to bind a protease on the non-structural protein 3, which serves as a part of the viral replication/transcription complex. This binding prevents the virus using its enzymatic activity to remove ubiquitin, allowing the host anti-viral interferon response to aid viral clearance. Other reviews discuss various proposed mechanisms of IVM in SARS-CoV-2 infection
Discussion
Ours and other data also support a protective role of Bifidobacterium in SARS-CoV-2 infection, possibly through these immune functions of Bifidobacterium. Our study and others show that the gut microbiome, particularly Bifidobacterium levels, relates to positivity and severity of SARS-CoV-2 infection. showed that changes in gut microbiota composition might contribute to SARS-CoV-2-induced production of inflammatory cytokines in the intestine, which may lead to the cytokine storm onset.
An increase in Bifidobacterium levels can reduce inflammation levels and TNF-α function, thereby calming the cytokine storm of SARS-CoV-2 infection. Bifidobacterium has been shown to bind TNF-α. This binding will absorb TNF-α from the gut, which, in turn, will reduce it in the blood stream, and eventually absorb it from the lungs and other affected areas (the “gut-lung axis”;
IVM is known to have antibacterial affects against Staphylococcus aureus and other gram-positive bacteria, which may seem contradictory to its potential to promote growth of another gram-positive bacterium, Bifidobacterium. However, a study by showed that certain Bifidobacterium spp. can have protective affects against S. aureus infection in mice, thereby acting in an antagonistic relation with S. aureus. Thus, both IVM and Bifidobacterium act against S. auereus, and it is unlikely that IVM would also act against Bifidobacterium.
If this presented hypothesis is true, the timing of IVM administration should be just prior to or at the cytokine storm. Seriously affected SARS-CoV-2-infected patients develop a cytokine storm alongside hypoxemia around Days 10–14, referred to as the “Second week crash” “Second-week crash” is time of peril for some patients with COVID-19). Our study on IVM combination therapy initiated therapy around Day 10, as patients typically presented to the study hypoxic at Day 9 (mean time from the symptom onset to treatment initiation was 9.2 days). This timing resulted in successful treatment, with all 24 severely hypoxic patients, recovering without hospitalizations. In short, IVM should typically be administered at the point of an SpO2 drop, the cytokine storm onset, and/or approximately Days 10–14.
Conclusion
We are hypothesizing the IVM mechanism of action as a therapeutic for COVID-19 is through feeding of Bifidobacterium, which then inhibits cytokine function and tames the cytokine storm . As such, IVM should be administered at the time of the cytokine storm.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
SH declares that she has a pecuniary interest in Topelia Pty Ltd in Australia, and Topelia Pty Ltd in United States where the development of COVID-19 preventative/treatment options are being pursued. She has also filed patents relevant to Coronavirus treatments. She is the founder and owner of Microbiome Research Foundation, ProgenaBiome, and Ventura Clinical Trials.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Sonya Dave, provided medical writing services for this manuscript and was funded by ProgenaBiome, LLC (The author’s institution). Thomas J. Borody, provided feedback on the manuscript.