Novavax Inc.’s vaccine was 90% effective at preventing Covid-19 in its pivotal trial, but the performance came before the emergence of the Omicron variant that has eluded vaccines more than earlier strains, U.S. health regulators said.
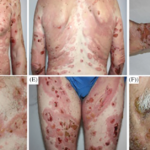
The Food and Drug Administration also expressed concern Friday that six people developed heart-inflammation conditions known as myocarditis and pericarditis, out of about 40,000 people who had taken Novavax’s vaccine during various studies.
This headline-only article is meant to show you why a stock is moving, the most difficult aspect of stock trading. Every day we publish hundreds of headlines on any catalyst that could move the stocks you care about on Benzinga Pro, our flagship platform for fast, actionable information that promotes faster, smarter trading.
Benzinga Pro has an intuitively designed workspace that delivers powerful market insight, and is the solution of choice for thousands of professional and retail traders across the world.
Stop Googling for information and check out Benzinga Pro. You will never again be left in the dark on when a stock moves. You’ll have what you need to act in real-time — before the crowd.