
In response to a Freedom of Information Request submitted by Children’s Health Defense, the Centers for Disease Control and Prevention last week admitted it never analyzed the Vaccine Adverse Event Reporting System for safety signals for COVID-19 vaccines.
In a stunning development, the Centers for Disease Control and Prevention (CDC) last week admitted — despite assurances to the contrary — the agency never analyzed the Vaccine Adverse Event Reporting System (VAERS) for safety signals for COVID-19 vaccines.
The admission was revealed in response to a Freedom of Information Act (FOIA) request submitted by Children’s Health Defense (CHD).
In September 2021, I published an article in The Defender in which I used the CDC’s published methodology to analyze VAERS for safety signals from COVID-19 vaccines.
The signals were loud and clear, leading me to wonder “why is nobody listening?”
Instead, I should have asked, “Is anybody even looking for them?”
After that article was published, I urged CHD’s legal team to submit a FOIA request to the CDC about its VAERS monitoring activities.
Since CDC officials stated publicly that “COVID-19 vaccine safety monitoring is the most robust in U.S. history,” I had assumed that at the very least, CDC officials were monitoring VAERS using the methods they described in a briefing document posted on the CDC website in January 2021 (and updated in February 2022, with minor changes).
I was wrong.
The lynchpin of their safety monitoring was to mine VAERS data for safety signals by calculating what are known as proportional reporting ratios (PRR’s).
This is a method of comparing the proportion of different types of adverse events reported for a new vaccine to the proportion of those events reported for an older, established vaccine.
If the new vaccine shows a significantly higher reporting rate of a particular adverse event relative to the old one, it counts as a safety signal that should then trigger a more thorough investigation.
The briefing document states, “CDC will perform PRR data mining on a weekly basis or as needed.”

And yet, in the agency’s response to the FOIA request, it wrote that “no PRRs were conducted by CDC. Furthermore, data mining is outside of the agency’s purview.”
The agency suggested contacting the U.S. Food and Drug Administration (FDA), which was supposed to perform a different type of data mining, according to the briefing document.

CDC officials repeatedly claimed they have not seen safety signals in VAERS.
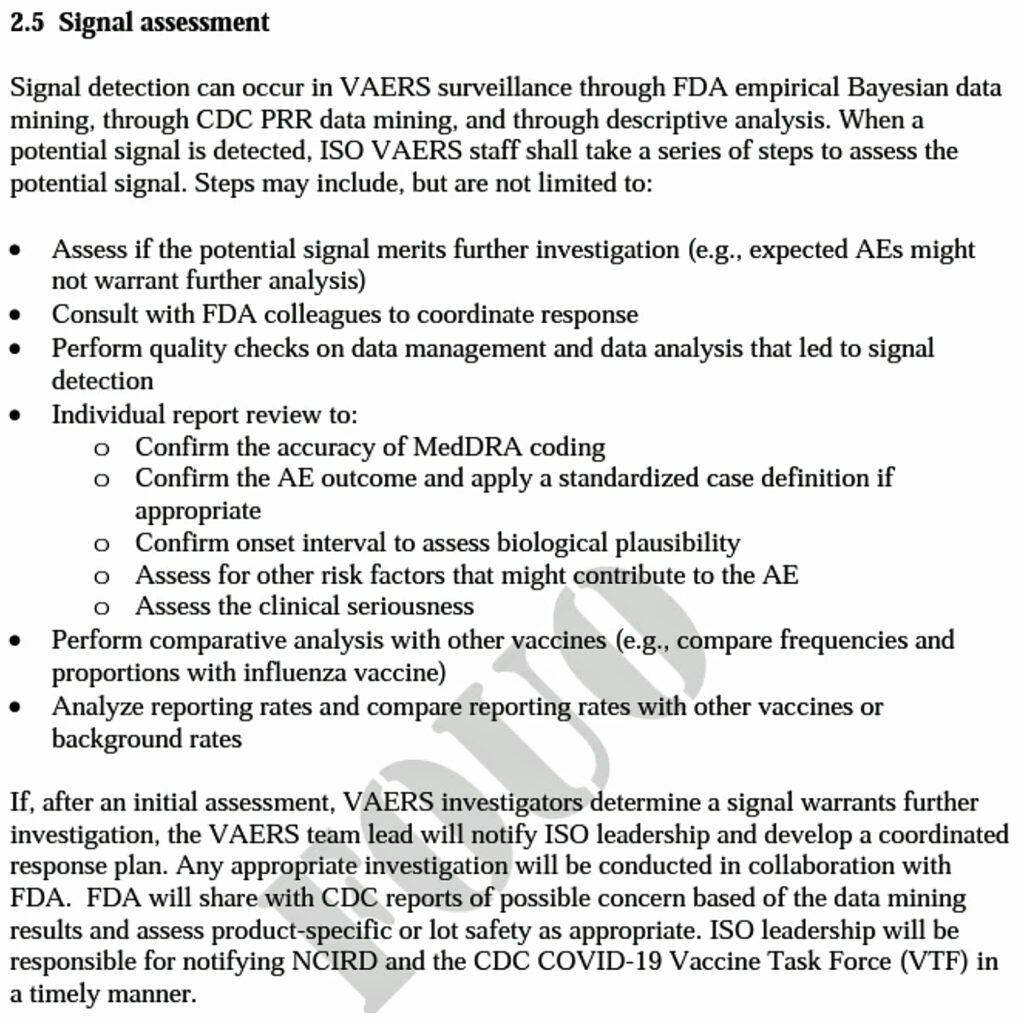 For example, on April 27, 2021, CDC Director Dr. Rochelle Walensky stated the CDC did not see any signals related to heart inflammation.
For example, on April 27, 2021, CDC Director Dr. Rochelle Walensky stated the CDC did not see any signals related to heart inflammation.
But a PRR calculation I did using the number of myo/pericarditis reports listed in the first table produced by the CDC obtained via the FOIA request reveals clear and unambiguous safety signals relative to the comparator vaccines mentioned in the briefing document (i.e., flu vaccines, FLUAD and Shingrix).
The table is dated April 2, 2021, almost four weeks before she made those remarks.
In fact, among the 15 adverse events for adults included in that week’s tabulations, PRRs I calculated also show loud-and-clear safety signals for acute myocardial infarction, anaphylaxis, appendicitis, Bell’s palsy, coagulopathy, multisystem inflammatory syndrome in adults (MIS-A), stroke and death.
The actual monitoring the CDC did diverges from the one promised in the briefing document in other ways.
For example, the CDC never created tables of the top 25 adverse events reported in the previous week, tables comparing different vaccine manufacturers, or tables of auto-immune diseases.
And it only began monitoring in early April 2021, even though reports from COVID-19 vaccines had been flooding VAERS since mid-December of the previous year.
To be clear, VAERS is not the only database the CDC uses to monitor COVID-19 vaccine safety.
For example, the CDC sponsored several studies of COVID-19 safety using the Vaccine Safety Datalink (VSD), which is comprised of millions of medical records from HMO’s across several states.
Those studies do not raise many safety concerns. However, they make many questionable methodological choices.
To give one example, a major safety study based on VSD data published in September 2021, in “JAMA,” compares adverse event rates that occur within 1-21 days of vaccination to the rate of occurrence from 22 to 42 days after vaccination.
It makes no comparison between vaccinated and unvaccinated individuals, or before vaccination versus after in the same individuals.
Moreover, the VSD is far from infallible, having failed initially to detect the increase in myocarditis rates.
In contrast, although calculating PRR’s is a blunt pharmacovigilance tool and far from perfect, it nevertheless has the advantage of being straightforward and difficult to manipulate with statistical sleight of hand.
PRRs are one of the oldest, most basic and most well-established tools of pharmacovigilance. The calculations are so straightforward that the CDC automated it several years ago, so it could have been done at the press of a button.
It simply beggars belief that the CDC failed to do this simple calculation. Even now, a paper published by CDC staff in March on the safety of the mRNA COVID-19 vaccines remains purely descriptive with no PRR calculation.
Meanwhile, a study published by a researcher not affiliated with the CDC in February in “Frontiers in Public Health” analyzes VAERS and EudraVigilance data using a method similar to PRRs, revealing clear and concerning safety signals.
And while it is true that VAERS is not the only database the CDC can use to monitor COVID-19 vaccine safety, it is of critical importance because it can reveal signals much faster than any other method — if anybody cares to look for them.
It remains to be seen if the FDA was properly monitoring VAERS. That will be the subject of a future FOIA request.
But even if it was, it doesn’t change the fact that the CDC completely failed in its promise to monitor VAERS for safety signals.
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the views of Children’s Health Defense.
FACUI: “There was no incidents of myocarditis or multi-system inflammatory syndrome of children.”
It seems “Mr. Science” missed these studies from the many available:



