The clinical trial of Pfizer’s mRNA “vaccine” did not prove the mRNA injection is safe and effective, despite Pfizer’s claims to the contrary. In fact, Pfizer stopped collecting useful data long before the planned end date of the clinical trial. Based on an inaccurate diagnostic test confirming COVID-19 in a tiny fraction of the study population, Pfizer researchers unblinded the control group and injected them with Pfizer’s mRNA “vaccine.” Because of these flaws in Pfizer’s clinical trial, no valid conclusions can be drawn about safety and effectiveness of Pfizer’s mRNA injection.
Pfizer researchers originally designed the clinical trial using the gold standard for drug testing: a double-blind, randomized, controlled trial. In this type of trial, neither patients nor researchers know who receives the drug (or intervention) being tested and who receives a placebo (double-blind). Researchers randomly choose trial participants to receive either the drug or placebo (randomized). Finally, researchers compare results of those who received the drug being tested (the experimental group) to those who received the placebo (the control group).
The Phase 3 randomized, controlled trial is the accepted standard method for assessing whether there is likely a beneficial effect due to an intervention (efficacy of the intervention). Randomized controlled trials “are the most stringent way of determining whether a cause-effect relation exists between the intervention and the outcome” (Kendall 2003, https://emj.bmj.com/content/20/2/164).
Pfizer initiated a Phase 3 trial on July 27, 2020, which enrolled more than 40,000 adults (age 16 and older). About half of the participants received two doses of the Pfizer mRNA injection, and the other half received two doses of a saline placebo. According to the trial protocol, Pfizer expected participants in both groups to continue for up to 26 months (p. 15, Clinical Protocol, https://cdn.pfizer.com/pfizercom/2020-11/C4591001_Clinical_Protocol_Nov2020.pdf), regardless of when various predetermined endpoints were reached.
Clinical trials typically involve both safety and efficacy endpoints. The safety of an intervention refers to the absence or low incidence rates of harmful side effects (adverse events). Efficacy endpoints refer to measurable outcomes, such as the rate of disease incidence (efficacy in the research world is generally equivalent to effectiveness in the real world). For a vaccine trial, researchers hypothesize that the disease rate in the experimental group will be significantly lower than the rate in the control group, based on a predefined expected difference.
As an endpoint, vaccine efficacy measures the reduction in disease rates (unvaccinated rate minus vaccinated rate) relative to the rate in the unvaccinated group. The Pfizer trial was designed to observe enough cases to provide a sufficient chance of detecting a minimal vaccine efficacy rate. Researchers can sometimes stop a trial early if the safety endpoint is clearly not being achieved and is thereby subjecting trial participants to high risks, or if the relative efficacy of the intervention is much greater than expected and can be established sooner than anticipated (that is, with fewer subjects). If researchers stop a vaccine trial early for reasons of efficacy, they may not have gathered sufficient data to establish the safety of the vaccine across various safety endpoints.
Pfizer designed its clinical trial to continue until 164 cases of COVID-19 were confirmed in trial participants after their second dose of Pfizer’s mRNA injection. These 164 cases would “be sufficient to provide 90% power to conclude true VE [vaccine efficacy] >30% with high probability” (p. 38, Clinical Protocol, https://cdn.pfizer.com/pfizercom/2020-11/C4591001_Clinical_Protocol_Nov2020.pdf). The US Food and Drug Administration (FDA) accepted this endpoint when it approved Pfizer’s original protocol.
Pfizer declared Phase 3 of the trial a success on November 18, 2020, after 170 confirmed cases of COVID-19 and just about four months after the trial began. In a press release, Pfizer announced, “Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints” (emphasis added). After just 170 cases of COVID-19 in 41,135 participants who had received their second doses by November 13, 2020, Pfizer called the efficacy test a success based on a COVID-19 incidence rate of only 0.4% (170/41,135).
To make matters worse, Pfizer researchers diagnosed these COVID-19 cases using the faulty polymerase chain reaction (PCR) test (p. 55, Clinical Protocol, https://cdn.pfizer.com/pfizercom/2020-11/C4591001_Clinical_Protocol_Nov2020.pdf). Researchers knew at the time that this test was inaccurate and had high rates of both false negative and false positive results. Yet the FDA approved PCR use in this clinical trial. Because most of these questionable COVID-19 cases were in placebo recipients, Pfizer declared the mRNA injection effective in preventing COVID-19, and the FDA approved the Pfizer injection for emergency use (EUA) on December 11, 2020.
At the same time, Pfizer and the FDA knew that some clinical trial participants had reported serious side effects from the mRNA injection. On November 24, 2020, Pfizer informed the FDA of the trial results in an interim report. Those results included 285 serious adverse events such as heart, liver, and neurological disease; cancers; and deaths. At that point, all trial participants were still blinded, and Pfizer could have continued the gold standard conditions for drug testing. Perhaps Pfizer and the FDA preferred to end the trial before more reports of serious adverse events could be recorded?
Meeting a milestone does not necessarily mean the end of a clinical trial. Yet, having reached the efficacy endpoint, in December 2020 Pfizer unblinded the placebo group and offered the mRNA injection to original placebo recipients (p. 4, Interim Protocol, https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf). By March 2021, nearly 90% of the original placebo group had received at least one dose of the Pfizer mRNA injection (p. 3, Safety Tables, https://phmpt.org/wp-content/uploads/2023/01/125742_S38_M5_c4591001-508-safety-tables.pdf). Thus, Pfizer effectively lost the ability to assess safety of the mRNA injection in comparison to a true control group.
Without a control group, data on adverse events are very difficult to interpret correctly, especially if one identifies only the original placebo and experimental (“vaccine”) groups without considering what happens to the placebo group after they receive the mRNA injection. If someone originally receives a placebo, later receives the mRNA injection, and then has a serious side effect, how is this event counted—as occurring in the placebo group or in the experimental group? If the event is counted among the placebo recipients, then potential harms caused by the mRNA injection are masked, making it difficult to get a true picture of the cause-effect relation.
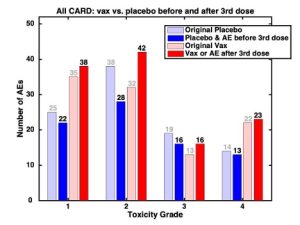
Those reporting the data must pay close attention to the date a placebo recipient received the mRNA injection (dose 3) and the date they experienced side effects. For example, Fig. 1 shows cardiac events in the original placebo (light blue) and original “vax” (light red) groups. When these groups are adjusted (dark blue and dark red) to account for cardiac events in the unblinded placebo recipients after they received the mRNA injection (dose 3), cardiac events in the original placebo group (light blue) are shifted to the adjusted “vax” group (dark red). Thus, the adjustment increases cardiac events in the “vax” group; conversely, cardiac events in the placebo group decrease when the onset date of the adverse event is determined to be after the placebo recipient was unblinded and given the mRNA injection (dose 3).
Pfizer graded these cardiac events as least serious (toxicity grade 1) to most serious (toxicity grade 4). In all grades, the effect of unblinding the placebo group was the same (Fig. 1). In all but grade 3, those who received the mRNA injection had more cardiac events than those who received the placebo (for grade 3, events in both groups were equal).
Pfizer sent a second interim report on these and other serious medical events to the FDA on April 1, 2021 (p. 77, Table 16.2.7.4.1, Interim Adverse Events, https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-interim-mth6-adverse-events.zip). When Pfizer presented these data, did they distinguish between the original placebo group and the unblinded, mRNA-injected placebo group? When FDA personnel reviewed this report, did they consider that most of the original placebo group had already received at least one dose of the Pfizer mRNA injection?
Why did the FDA authorize emergency use of Pfizer’s mRNA injection based on COVID-19 diagnosis in such a small fraction (0.4%) of the total study population? Why did the FDA approve the inaccurate PCR test for use in diagnosing COVID-19? Why did the FDA allow Pfizer to unblind the control group and abandon the gold standard in its clinical trial even as more and more mRNA recipients were seriously injured every day?
To Pfizer and the FDA, the clinical trial was a success; a “historically unprecedented achievement” according to Pfizer’s press release. But when Pfizer unblinded the placebo recipients and ruined the control group, the clinical trial of Pfizer’s mRNA injection did, in fact, fail. The ability to gather conclusive evidence of the long-term effects of Pfizer’s mRNA injection was destroyed, and no valid conclusions can be drawn about safety and effectiveness from Pfizer’s flawed clinical trial. When will Pfizer and the FDA admit this failure and stop promoting the mRNA “vaccine” as safe and effective?
Fig. 1 Cardiac adverse events (AEs) adjusted for placebo group unblinding (dose 3)
Report Summary
Most important finding: The clinical trial of Pfizer’s mRNA “vaccine” failed, despite Pfizer’s claims that the mRNA injection has been proven to be safe and effective.
Key detail leading to finding: Pfizer researchers deliberately stopped collecting data on the control group soon after the trial began.
Events of concern: Pfizer stopped the trial
- long before the planned end date
- when only a small fraction of participants had contracted COVID-19
- after COVID-19 diagnoses made based on inaccurate PCR tests
- while other participants were still reporting serious side effects
Further investigation: Why did the FDA authorize emergency use of Pfizer’s mRNA injection based on COVID-19 infection in a small fraction (0.4%) of the total study population? Why did the FDA approve the flawed PCR test for use in diagnosing COVID-19? Why did the FDA allow Pfizer to unblind the control group and abandon the gold standard in its clinical trial even as more and more mRNA recipients were seriously injured every day? When Pfizer presented subsequent data, did they distinguish between the original placebo group and the unblinded, mRNA-injected placebo group? When FDA personnel reviewed subsequent data, did they consider that most of the original placebo group had already received at least one dose of the Pfizer mRNA injection?
Scale of situation: The FDA approved Pfizer’s mRNA injection based on a flawed clinical trial.
Explanation of key scientific term: A double-blind randomized controlled trial is an experiment in which neither patients nor researchers know who receives the drug (or intervention) being tested and who receives a placebo (double blind); the trial participants are randomly chosen to receive either the drug or placebo (randomized); and the results of those who received the drug being tested (the experimental group) are compared to those who received the placebo (the control group).