
In the year since he became partially paralyzed, Ross Wightman has kept his focus on small victories — from getting up the stairs unassisted, to going for a solo walk near his rural B.C. home.
But the biggest win came in the form of an e-mail from Canada’s Vaccine Injury Support Program (VISP) that confirmed something he says he knew all along: that his condition was likely caused by the Oxford-AstraZeneca COVID-19 vaccine.
“That was quite vindicating,” Wightman said from his Lake Country home in the Okanagan Valley. “To have it in hand, in paper, acknowledging it has been vindicating.”
Wightman was diagnosed with Guillain-Barré Syndrome (GBS), a rare condition that affects the nervous system, just days after his first and only dose of the vaccine. The condition can cause paralysis, muscle weakness, and even death.
“Every day is a grind,” said Wightman, who still has substantially limited mobility in his arms and legs. “[The letter] doesn’t change my condition, or the way I feel overly — it’s just nice to have,” he added.
GBS diagnoses following a COVID-19 vaccination are extremely rare — about one in 700,000 — according to data from the B.C. Centre for Disease Control (BCCDC) and Health Canada.
There have been 10 reports of individuals hospitalized with GBS within 30 days of a COVID-19 vaccine since December 2020, all of whom have been discharged, according to the BCCDC. Four reports followed the AstraZeneca vaccine, five followed Pfizer-BioNTech Comirnaty, and one followed Moderna Spikevax.
More than 11.7 million doses of the COVID-19 vaccine have been administered in B.C., with health experts noting the risks associated with coronavirus infection far outweigh the risks of vaccination. There have been more than 41,000 deaths associated with COVID-19 in Canada.
The letter Wightman received makes him one of just a handful of Canadians to become approved for a COVID-19 vaccine injury benefit. He chose not to share his total allotment with CBC News citing privacy concerns, but said the maximum payout under the program is about $284,000. Wightman said he did not qualify for the full amount.

He said he is also eligible for income replacement up to $90,000 per year.
CBC News has contacted Health Canada for clarity on the payout structure of the program. Its most recent data suggests fewer than five people have been approved for the injury benefit, with numbers to be updated in the next few days, program operators say.
The operators also said Wednesday that the latest vaccine injury data will be released at a later date despite its own June 1 deadline.
‘Not overly excited’
Wightman, who worked as a pilot and real estate agent prior to his diagnosis, has spent the last year unable to work. He can’t travel far on his own. But the hardest thing is sitting on the sidelines, unable to do physical activities with his kids, he says.
“Playing soccer with the kids in the yard. My oldest is big into baseball, and one of my favourite things to do is just play catch in the yard, and I can’t do that. That’s hard,” he said.
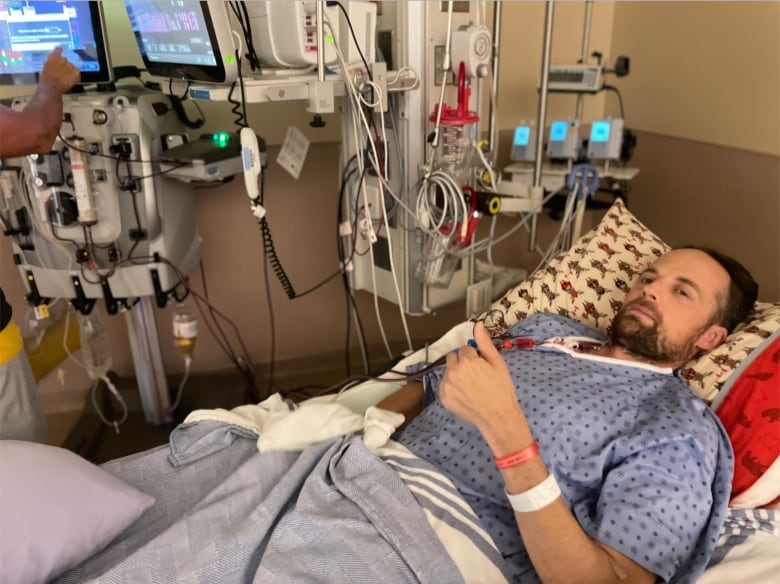
Despite his approval of vaccine injury payout, he says he still doesn’t think it matches the physical, emotional and financial toll he’s endured over the past year.
“I don’t know what number I can say is enough, but [the payout] is not something that I’m overly excited about,” he said. “The income replacement won’t be what we’ve been used to … so that’s a little disappointing to me.”
Wightman says a number of his symptoms, including loss of feeling in his feet and vision impairments, weren’t included in his injury benefit assessment, so he plans to appeal to the program’s medical review board. He says he’s also seeking legal advice.

“If the lump sum was huge, then maybe the income replacement is something we could make work, but the way things have been presented so far, it’s not something we’ve decided on yet,” he said.
Vaccine compensation
Prior to the pandemic, Canada was the only G7 country that did not have a vaccine injury compensation program.
The country’s mass COVID-19 immunization plan spurred the development of the VISP, said Dr. Kumanan Wilson, an internal medicine physician at Ottawa Hospital who was consulted as a subject matter expert for the program. Wilson is also the CEO of CANImmunize, the tech company behind the digital vaccine tracking platform of the same name, and an expert in vaccine hesitancy.

“We did tell people you need to get vaccinated, and in many cases mandates were brought in,” he said. “We needed to hold up our end of the bargain, and that was making sure that these individuals were treated fairly if something untoward were to happen.
“I’m a very strong believer in the safety of vaccines. They go through rigorous phase three trials, but rare events can happen, and in those circumstances, those individuals need to be supported,” he added.
Wilson notes risks of COVID-19 infection far outweigh the risks of adverse reactions to vaccines. The AstraZeneca vaccine was largely phased out in Canada after blood clots appeared in recipients at a rate of about one in 100,000.
Wilson said there were challenges early on in the development of the program determining what should be considered a serious illness that’s associated with a vaccine. GBS was among the disorders discussed, given that it comes with severe health challenges that can often be overcome after a number of years.
“I’m encouraged to see that condition was compensated, that they erred on the side of a liberal interpretation of serious and permanent harm,” he said.
As to whether or not recipients will be happy with the assessed payouts from the program, Wilson said they can exercise their right to appeal, while noting that VISP is still in its early stages.
“There will likely be a lot of adaptations made based on the experiences of the initial claims.”



