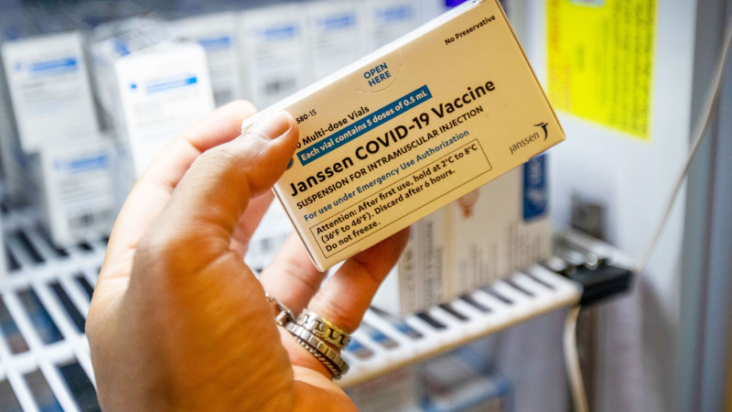
🇺🇸💉@COVID19Up: The U.S. FDA announced (https://www.cnbc.com/2022/05/05/fda-limits-use-of-jjs-covid-19-vaccine-for-adults.html) on Thursday (May 5) that it was strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to the risk of a potentially life-threatening side effect.
The agency said it has now limited the use of the vaccine to individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate.
The FDA said its analysis had determined that the risk of thrombosis with thrombocytopenia syndrome after the administration of the injection warrants limiting of the authorization.
Johnson & Johnson did not immediately respond to a Reuters request for comment.